Brief Overview of (f)NIRS
Concept Knowledge
In order to identify neural correlates triggered by specific HRI collaborative tasks, the project must resort to technology capable of probing neural activity. Here is where Brain-Computer Interfaces (BCI) come in. While most approaches typically rely on electroencephalography (EEG), this does not enable enough spatial granularity for activation to be associated with specific brain regions. Moreover, we still require BCI technology to be non-intrusive and non-cumbersome towards collaborative tasks. Thus, functional near-infrared spectroscopy (fNIRS) comes in handy.
Unlike EEG, fNIRS technology uses optode transmitters and receivers, to measure light loss resultant from propagation in soft tissue. Different events, such as scattering and absorption, induce variation in the light travelling from emitter to receiver, and thus may be correlated with substance concentration in those tissues. More specifically, oxyhemoglobin Δ[𝑶2𝑯𝒃] and deoxyhemoglobin Δ[𝑯𝑯𝒃] concentrations are estimated over time, via their respective chromophores, in the surface area of the cerebral cortex where a cap with optodes is placed. By assuming tissue to remain unchanged, a modified Lambert-Beer law may be employed for such estimates.
The idea behind concentration monitoring comes from the fact that, when neurons are active, they consume more oxygen. This factor leads to a local increase in deoxyhemoglobin and decrease in oxyhemoglobin. Consequentely, a signal can be considered, indicating the site and level of neural activity. To this end, optode transmitter-receiver arrangement and pair quantity dictate the number of channels on which concentrations are being estimated, thus influencing the granularity of the activation mapping being generated.
Pair distance is an impactful factor in fNIRS, as it determines the depth of brain tissue being measured and the proportion of the signal that comes from the scalp versus the brain. Here, distances are kept short (30mm), for cortex monitoring. Finally, optode arrangement should be placed over the cortical area meant to be monitored (e.g. prefrontal cortex or PFC).
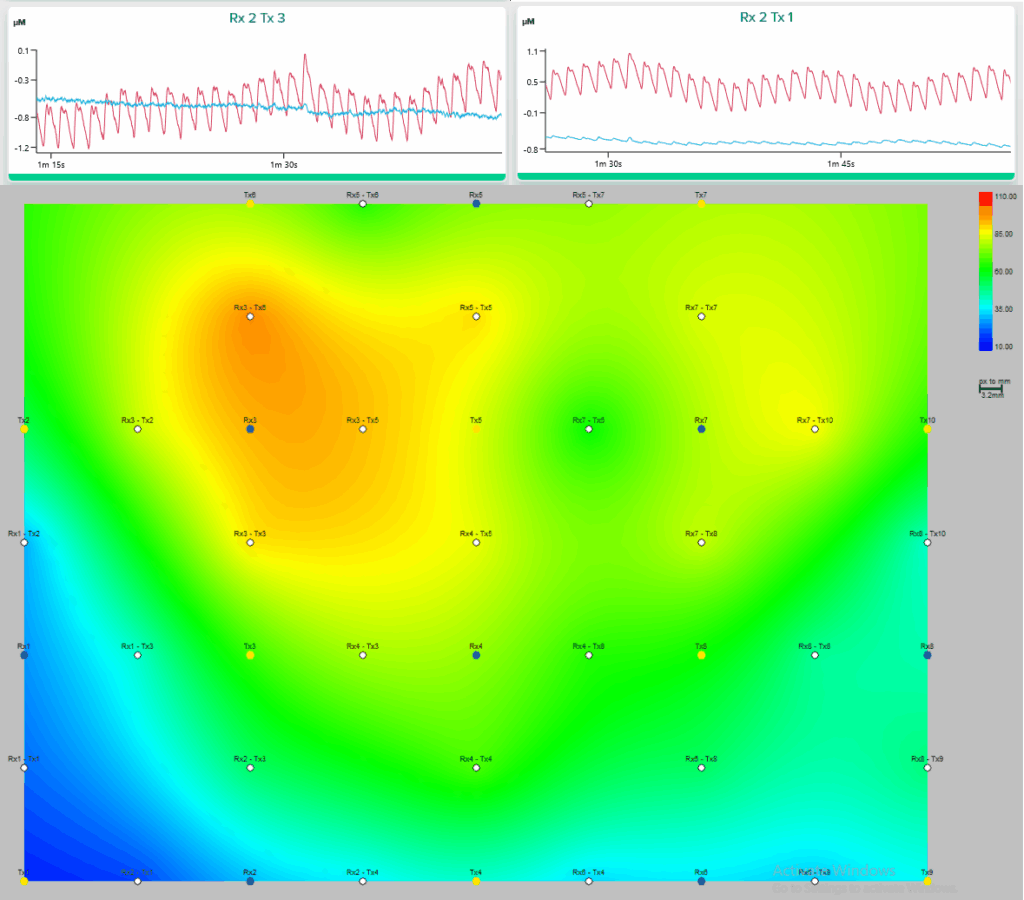
For each channel available, deoxyhemoglobin and oxyhemoglobin variation over time can be estimated, and thus a corresponding graph may be obtained. This is particularly useful when monitoring for localized activity, where the channel is placed. Evidently, when considering multi-channel arrangements, then a wider topographic mapping may be obtained for cortical activity, considering the localized variations of those concentrations. To learn more about fNIRS and the theory behind this technology, we suggest visiting Artinis’ post and suggested papers on the topic.
In CoDRI, this level of cortical activation knowledge can be extremely useful as it pinpoints which area is undergoing more variation, while ergonomic factors of human-robot interaction fluctuate. Thus, correlations between spatial activation and ergonomic variation can be extracted, based on which appropriate feedback can then be generated for robots to adapt their demeanours.
All this is possible as fNIRS provides enough temporal and spatial resolution, without hindering user mobility. Unlike techniques which typically explore brain activity in a spatial manner yet restrict the user, such as fMRI, fNIRS caps enable other activities to be performed, while signal is being acquired, thus making it ideal for collaborative robotics tasks.